Published on
Published 11/3/2023
Teresa Lever is developing tools to objectively diagnose and treat swallowing impairments associated with neurological diseases, cancer, genetic disorders and aging.
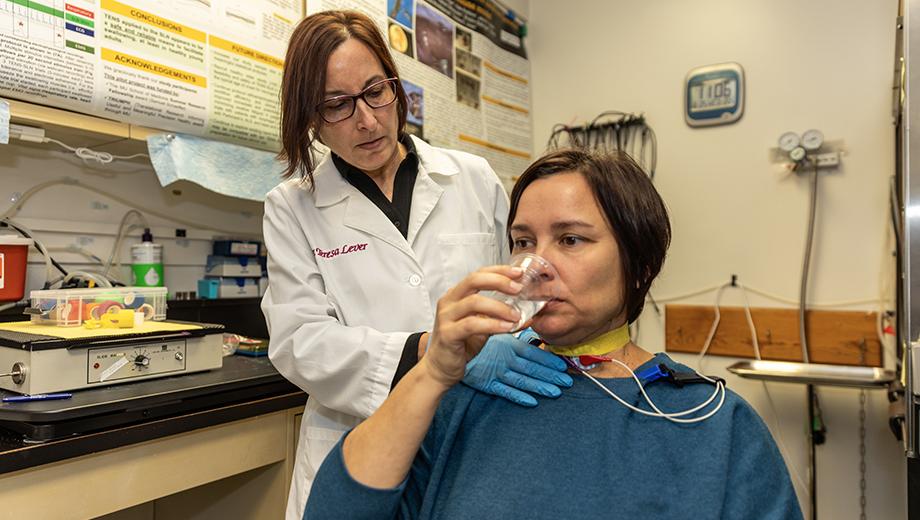
Dysphagia, or difficulty swallowing, affects about 15 million adults in the United States, according to the National Foundation of Swallowing Disorders. Those with the condition are unable to eat and drink normally and often suffer from depression, malnutrition and an increased risk for life-threatening aspiration pneumonia.
Teresa Lever, associate professor in the School of Medicine’s Department of Otolaryngology – Head and Neck Surgery, is a clinician-scientist who specializes in the diagnosis and treatment of adult-onset neurological disorders affecting the aerodigestive tract, the combined pathway for air and food essential for swallowing, breathing and speaking. She says her work overlaps with numerous fields, such as neurology, gastroenterology, pulmonology, internal medicine and radiology.
Funded by multiple grants from the National Institutes of Health (NIH) and National Science Foundation (NSF), Lever conducts interdisciplinary research with collaborators at the University of Missouri and other institutions in the U.S. and abroad to improve the quality of life for people and animals with dysphagia caused by a stroke, Alzheimer’s disease, Parkinson’s disease, amyotrophic lateral sclerosis (ALS), cancer, genetic syndromes and other disorders.
“Current dysphagia treatments are predominantly palliative rather than curative because the underlying pathological mechanisms remain largely unknown for each condition,” Lever says. “Dysphagia diagnosis and treatment response monitoring are heavily reliant on subjective rating scales that are, unfortunately, prone to clinician bias and often cannot detect clinically meaningful changes in the patient’s swallowing function.”
Tell us about your current research goals.
My research is focused on dysphagia, with a secondary focus on other aerodigestive tract functions (i.e., breathing, voice production and speaking). Specifically, I study the neural control and neuropathology of these functions. My goals are to:
- Develop diagnostic tools and methods for objective detection, tracking and monitoring of dysphagia across species.
- Establish additional rodent models of dysphagia to study the causative mechanisms, which are likely different for each disease and condition. While numerous rodent models of neurological and other conditions currently exist, they have rarely been studied relative to dysphagia. So far, I have developed rodent models of dysphagia for ALS and other motor neuron diseases, DiGeorge syndrome and other neurodevelopmental disorders, oropharyngeal cancer and radiation treatment effects, and senescence or advanced biological aging.
- Develop effective, targeted treatments for dysphagia in rodent models to implement in clinical trials with human and veterinary patients. Current dysphagia treatments are largely behavior-based and, therefore, do not target the underlying pathological mechanisms.

How are new tools and animal models helping your team pioneer new treatments?
Using our rodent models of dysphagia, my research team has been investigating numerous therapeutic candidates with high translational potential for human and veterinary patients, including a variety of pharmacological, electrophysiological, genetic/optogenetic and behavior-based approaches in mice and rats. The goal of these targeted treatments is to delay, slow, halt or reverse dysphagia symptoms, which we can now detect and measure objectively using our new tools and methods.
I also am adapting treatments proven to be safe and effective in rodent models for use with human patients. The first such study (i.e., targeted laryngeal nerve stimulation to elicit repetitive swallowing) was recently completed in healthy young adults and is now being expanded into a multisite study with patients suffering from chronic dysphagia due to stroke, ALS, Parkinson’s disease and head and neck cancer. Additional experimental treatments that pass preclinical safety and efficacy studies in rodents will follow suit.
Why did you transition from patient care to translational research?
As a medical speech-language pathologist, I was frustrated by the numerous knowledge gaps and lack of objectivity in my field. It’s difficult to know if you have an effective treatment without the ability to quantitatively measure changes over time. That’s why I left clinical practice in 2002 to pursue a research doctoral degree focused on the neural control of normal swallowing and dysphagia and completed a postdoctoral fellowship, both at East Carolina University. In 2010, I joined the MU faculty with newly awarded NIH-R03 funding that I used to develop my research program.
How is your research solving problems for multiple species?
My focus is truly multidisciplinary and translational at its core. Aerodigestive disorders are quite common in companion animals. For this reason, my research program involves multiple species (e.g., humans, dogs, horses and rodents) and spans from basic science to veterinary medicine to clinical research. This “one health” approach embraces a collaborative effort between human and veterinary health communities to solve health-related problems.
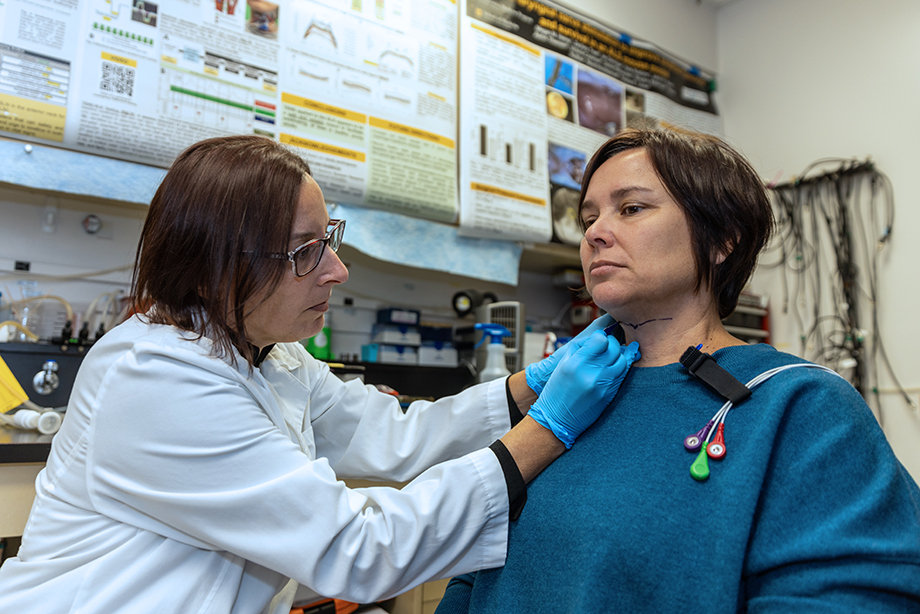
Describe a few highlights from your innovation journey.
I have developed several patented tools and methods that permit objective quantification of aerodigestive tract functions across species, and they are being used in numerous animal and human studies within and beyond MU. Currently, my lab is in the process of testing and validating the effectiveness of these new technologies in detecting, monitoring and treating aerodigestive tract disorders, including swallowing, breathing and speaking impairments in human and veterinary patients.
The end goal is to translate these biomedical innovations into clinician-friendly diagnostic and treatment tools that may directly improve human and veterinary healthcare outcomes. Two of my patents were recently licensed from MU to my startup company, Aerodigestive Health Corp. (formerly Lever Scientific). My plan in 2024 is to apply for NIH and NSF small business grants, which support research projects with marketplace potential. MU’s Technology Advancement professionals have been instrumental in helping me pave the way for the commercialization of future life-changing products for patients.
What is your teaching philosophy with students in your lab?
In both the classroom and research settings, the majority of my students are aspiring physicians, veterinarians and speech-language pathologists. My most challenging teaching role thus far entails research mentorship, which necessitates keeping students interested in learning amidst the typical slow pace and often tedious nature of research projects.
My training philosophy is to inspire students to critically think before they leap, communicate with enthusiasm and be rigorous and transparent in their research. When faced with challenges, which occur often in research, I encourage students to come to me with creative solutions to their identified problems, which we then tackle together. I spend an enormous amount of time working one-on-one and in small groups to ensure students succeed within and beyond the research setting. Nothing is more gratifying than hearing from students after graduation that I was one of the people who contributed to their academic and life achievements.