Etiology: Mycoplasma pulmonis is a pleomorphic bacterium lacking a cell wall. This organism can induce a chronic pulmonary disease syndrome. Cilia-associated respiratory (CAR) bacillus is a frequent co-pathogen with M. pulmonis.
Incidence: The incidence of infection remains common in non-SPF colonies [1].

Transmission: Transmission occurs by direct contact with infected secretions. M. pulmonis can also be transmitted transplacentally. Rats may act as asymptomatic carriers for Mycoplasma pulmonis.
Clinical Signs: Infection begins without clinical signs. Adverse environmental factors such as high cage ammonia levels, and/or the acquisition of primary viral or bacterial respiratory pathogens can activate subclinical infections. Early signs of overt disease may include an oculonasal discharge and torticollis (see photo). Other clinical signs include snuffling, chattering, anorexia with weight loss, rough hair coat, hunched posture, and reduced fertility.
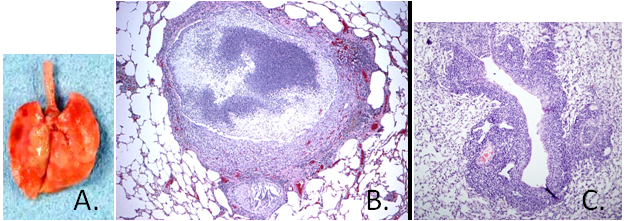
Pathology: In the upper respiratory tract, a purulent discharge may be found on the nasal mucosa and within the tympanic bullae. This purulent exudate can be found in the trachea and bronchi causing yellow parenchymous foci which may progress to form bullae (bronchiectasis) and red to grey areas of consolidation (A.). Histological examination of lungs reveals a purulent bronchopneumonia with bronchiectasis (B.) with moderate hyperplasia of peribronchial lymphoid aggregates (C.). Mycoplasma pulmonis does not stain with histochemical stains due to the absence of a cell wall.
Diagnosis: MFI and IFA are commercially available for serological screening for Mycoplasma pulmonis infections in mouse colonies. PCR of nasal or tracheal samples is also used for diagnosis.
1. The Mouse In Biomedical Research. 2 ed. American College of Laboratory Animal Medicine. Vol. 2. 2007, 30 Corporate Drive, Suite 400, Burlington, MA 01803: Elsevier.